Why ClO₂ Is Unique — Odor Control & Beyond
ClO₂ (chlorine dioxide) combines powerful oxidation with targeted action — delivering superior odor control and sanitizing performance.
Introduction: What Makes a Molecule “Unique”?
All matter — including the odors you notice — is made of molecules, the smallest identifiable units composed of atoms bound together. The behavior of a molecule depends on how its atoms share or exchange electrons.
Among thousands of molecules, chlorine dioxide (ClO₂) stands out for its unusual chemical structure and how it interacts with odor-causing compounds and microbes — making it ideal for deodorizing and sanitation.
1) Chlorine Dioxide Simplified: Stability + Selectivity
ClO₂’s molecular structure makes it distinct:
- It has an odd number of valence electrons, which is rare in stable molecules and gives ClO₂ special oxidative properties.
- It does not readily break down into chlorine gas, unlike many other chlorine-based oxidizers.
Why this matters:
- Many oxidizers react indiscriminately with everything they contact, leading to unwanted byproducts or damage.
- ClO₂’s electron configuration gives it a selective oxidative behavior, meaning it preferentially interacts with sulfur- and nitrogen-based odor molecules and biological contaminants — precisely the molecules that most often cause bad smells.
This selectivity is why ClO₂ is widely used in water treatment, industrial deodorization, and sanitation.
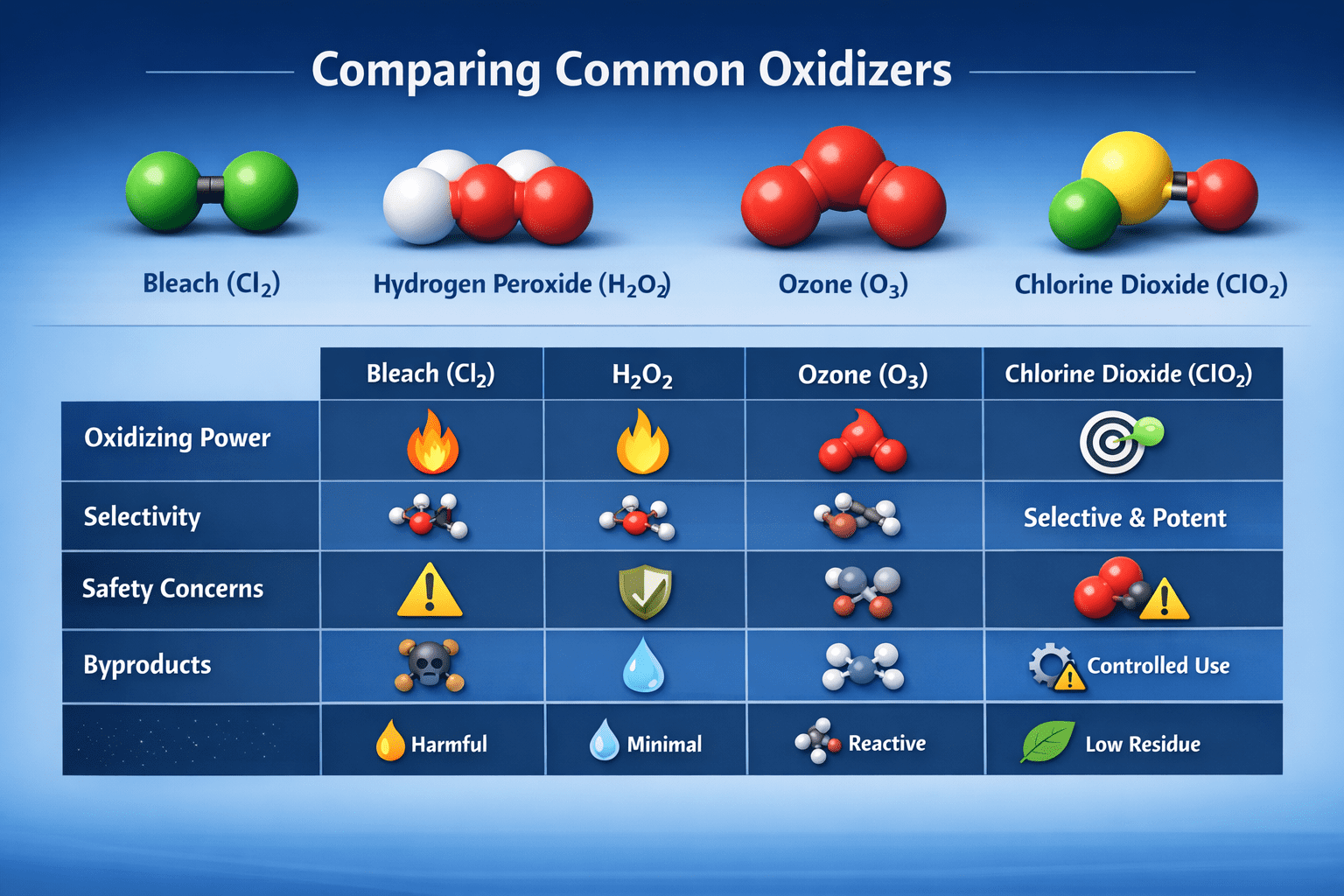
2) Comparing ClO₂ to Other Oxidizers
Here’s how ClO₂ stacks up against alternatives:
| Oxidizer | Common Use | Reaction Behavior | Typical Drawbacks |
|---|---|---|---|
| Bleach (Cl₂) | Household disinfection | Broad oxidation | Creates harmful disinfection byproducts (DBPs) |
| Hydrogen Peroxide (H₂O₂) | Surface cleaning | Moderate oxidizer | Less potent against stubborn odors |
| Ozone (O₃) | Air/purification | Strong oxidizer | Can be unsafe at effective concentrations |
| Chlorine Dioxide (ClO₂) | Deodorization & disinfection | Highly selective & powerful | Needs controlled use due to oxidizing strength |
ClO₂’s advantage: it produces fewer harmful byproducts than chlorine gas and is effective against microbes at low concentrations, while still maintaining powerful odor-neutralizing potential.
3) How ClO₂ Actually Eliminates Odor
Odors come from volatile organic compounds (VOCs) — molecules that release into the air when they interact with receptors in your nose. ClO₂ works by oxidizing these VOCs:
- ClO₂ pulls electrons from odor molecules.
- This electron extraction changes the molecular structure, neutralizing the smell at a chemical level.
- The result: the compound that smelled bad no longer exists in its original odor-causing form.
This process is more effective with ClO₂ because of its oxidation potential — which can be significantly higher on a per-molecule basis compared to other oxidizers used in odor control.
4) Safety & Best Practices
While powerful, ClO₂ must be used responsibly:
- In gaseous or highly concentrated forms, ClO₂ is a strong oxidizer and must be handled with proper controls, as required in industrial hygiene standards.
- For consumer deodorization products (like FreshTent kits), formulations are designed to produce effective concentrations that stay within safe use levels.
Always follow product instructions and safety guidelines printed on packaging. Here is a chart that shows how ClO2 compares with other Oxidizers:
5) FreshTent’s ClO₂ Products: What They Offer
When you use FreshTent’s ClO₂-based odor control solutions, you benefit from:
✔ Targeted oxidation of odor compounds
✔ Effective in air and on treated surfaces
✔ Broader sanitizing action on microbes that contribute to smells
✔ Fewer harmful byproducts vs. traditional chlorine
Our product lineup includes:
- Full Room Deodorizing Kit
- Small Space Deodorizing Kit
- 30-Day & 60-Day Deodorizing Granules
- Gel Deodorizing Kit
- Odor Control Cleaning & Deodorizing Starter Kits
Each is formulated to harness ClO₂’s properties in a practical, safe way for tents, RVs, boats, gear, and outdoor spaces.